Background: ITP management often requires subsequent therapy beyond current first line treatments (corticosteroids or intravenous immunoglobulin). The use of thrombopoietin receptor agonists (TPO-RAs) as second-line treatment, in lieu of splenectomy or rituximab, has become more common and is supported by recent American Society of Hematology (ASH) guidelines (Neunert, 2019).
The TPO-RAs eltrombopag (ELT) and romiplostim (ROMI) have been utilized in patients with ITP for over a decade, whereas avatrombopag (AVA) was more recently approved in June, 2019. Unlike ROMI, which is a weekly in-office injection AVA is an oral medication taken at home. ELT carries a boxed warning for hepatotoxicity and is a chelating agent. In order to mitigate clinically relevant effects on the pharmacokinetic profile from chelation, ELT must be administered two hours prior to, or four hours after meals containing polyvalent cations such as calcium or magnesium. AVA does not require liver function monitoring and has not demonstrated hepatotoxicity in studies. Additionally, AVA is administered with food, without food type restrictions, as it does not chelate polyvalent cations.
Thromboembolic events (TEEs) are not uncommon in ITP, with studies showing that up to 8% of patients experience an arterial or venous event (Sarpatwari, 2010; Vianelli, 2013). As agents that potentiate endogenous platelet production, TPO-RAs as a class may increase the risk of thromboembolism, with such events occurring in a variety of TPO-RA ITP studies. However, it is not well-understood what increased risk, over and above the inherent risk associated with ITP, TPO-RAs pose in regard to thromboembolic events (Catala-Lopez, 2015).
Aims: To characterize the TEEs occurring across the AVA ITP clinical development program.
Methods: 4 studies were conducted evaluating AVA in patients with ITP (Two Phase 2 and two Phase 3 trials). In total, 128 patients received AVA treatment, with an average duration of exposure of ≥180 days. Occurrence of TEEs was an adverse event of special interest that was monitored closely in these studies. At the time of each TEE, the following information was collected: platelet count, AVA dose, study day of event and other medical history and lifestyle factors potentially increasing the risk for thromboembolism. Patients with history of arterial or venous thrombosis and more than 2 significant risk factors were excluded from the Phase 3 studies.
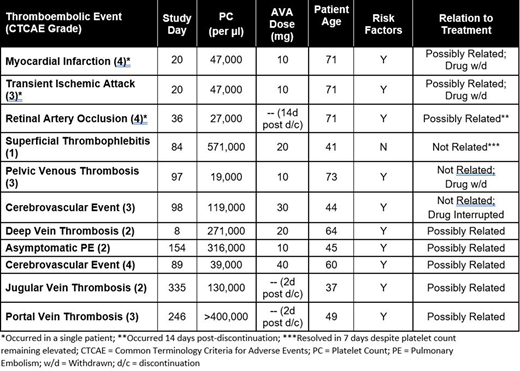
Results: A total of 11 TEEs occurred in 9/128 (7%) of AVA treated patients, with one patient experiencing 3 events. No clustering of events was noted, with cerebrovascular accident being the only specific event occurring in more than one patient (occurring in 2/11 patients). Variability was observed in the platelet count at the time of TEE, ranging from 19,000 - 571,000/µL. Two patients experienced events at a platelet count >400,000/µL, whereas five events occurred at a PC <50,000/µL. The onset of thromboembolic events ranged from study day 8 to 335, with no clear pattern materializing regarding duration of drug exposure and the onset of the TEE. 8 of the 9 patients experiencing thromboembolic events had other potential contributing factors in their medical history, including coronary and cerebrovascular disease, genetic predispositions such as Factor V Leiden, tobacco use, anti-phospholipid antibody positivity and malignancy. 3 of the 11 events were not deemed to be related to AVA treatment, whereas 8 events were considered possibly related to AVA. Thromboembolic events were noted at daily doses of AVA ranging from 10 - 40 mg. No deaths were noted in the ITP development program.
Conclusions: The tTEEs noted with AVA treatment typically occurred at PCs below the upper bound of normal (450,000/µL), without relationship to drug dose, at varying number of days on study drug, and often were associated with additional risk factors. No clear patterns regarding the occurrence of thromboembolic events with AVA treatment could be determined. Analyses of other TPO-RAs and TEEs have led to similar conclusions regarding a lack of relationship between platelet count and drug dose (Ghanima, 2018). Clinicians should carefully monitor PC and assess the risk for thromboembolism in each individual patient treated with a TPO-RA.
Piatek:Alexion Pharmaceuticals: Consultancy, Research Funding. Jamieson:Dova Pharmaceuticals: Current Employment. Vredenburg:Dova Pharmaceuticals: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal